2018 美国药典多肽药物质量与标准国际论坛
2018-04-19 至 2018-04-20
杭州
王艳红
13884941886
邀 请 函
2018美国药典多肽药物质量与标准国际论坛
USP International Therapeutic Peptides Quality and Standards Forum 2018
2018年4月19-20日 中国?杭州
April 19-20, 2018 Hangzhou, China
主办方 Organizer:美国药典委员会 (U.S.Pharmacopeia)
协办方 Co-organizer :世易科技 (eChinaChem)
会议介绍 Conference Introduction:
多肽指由氨基酸经过肽键连接在一起而形成的化合物,通常意义上超过四十个氨基酸的称为蛋白,而小于四十个氨基酸的称为多肽。多肽药物的发展由来已久,其范围可以囊括合成多肽,重组多肽甚至是胰岛素类产品。进入二十一世纪以后,随着生物技术以及生物医药的迅猛发展,多肽药物也得到快速发展,全球的多肽药物市场预计将会超过250亿美元。中国作为原料药生产大国,越来越多的企业开始进入多肽药物领域。但是,由于其分子量介于传统小分子化药与生物大分子药物之间,加之生产工艺的多样性,导致多肽药物的质量研究以及标准制定存在诸多困难,如何做好多肽药物的质量研究,进而确定相应的质量标准已成为业界最关心的话题之一。
美国药典委员会 (USP) 作为全球领先的药物质量标准制定机构,本次特别邀请海内外标准设定机构、法规监管、多肽研发、生产等专家共聚一堂,将与您共同交流和讨论多肽药物中国前景与机遇、GMP生产与法规、多肽药物质量研究的技术发展、多肽药物的药典标准更新以及杂质的分析技术和质控标准。
Peptide drugs have a rich history, covering both synthetic and recombinant peptides, and insulins. With the rapid development of biotechnology and biopharmaceuticals, peptide drugs also enjoyed rapid expansion, with global market expected to exceed $25 billion. China is a major global supplier for APIs, and more and more companies are getting into the peptides. However, due to unique properties of peptides, including molecular sizes between the small molecule chemical medicines and large molecule biopharmaceuticals, multiple manufacturing processes, there remain many challenges for the quality determination and standard-setting for peptide drugs, which is one of the hottest topics in the peptides community.
USP, as part of the global leading standard-setting organization for medicines, cordially invites experts, both domestic and overseas, from standard-setting organizations, regulatory agencies, peptides manufacturing, peptides research and development, to participate and gather for the international forum on therapeutic peptides quality and standards. The forum will provide the platform for conference attendees to present and discuss perspectives on current landscapes and future opportunities of peptide drugs in China, GMP manufacturing and regulations, state-of-the-art technologies for peptides quality, updates on peptides compendial standards, and analytical technologies and quality standards for impurities.
参会对象 Participants:
多肽(包括合成多肽、重组多肽、及胰岛素)原料药/制剂生产、研发人员;国际注册和法规事务人员;国际市场开发人员;质量负责人及其专员;多肽质量标准、法规监管人士;多肽质量和技术研究的学术机构/科研单位人员;以及其他对研讨会主题感兴趣的人员。
Peptides (including synthetic and recombinant peptides, and insulins) API/formulation manufacturing and R&D; international registration and regulatory affairs; international marketing; quality control and quality assurance; peptides standard-setting and regulatory; academic research involving peptides quality and analytical technologies; others interested in the topics of the international forum.
演讲嘉宾来自 Speakers:
1)USP
2)US FDA
3)China Regulatory Authority
4)Industry / Institutions / Associations
(详细演讲嘉宾名单和演讲主题将于第二轮通知呈上)
Detailed speaker list and presentation topics will be available in the next round announcement.
会议四大议题 Sessions:
议题一:多肽药物在中国的前景与机遇
Development and opportunities of therapeutic peptides in China
议题二:多肽药物及其相关杂质的分析表征与质控技术
Analytical techniques for peptide characterization, impurities and quality control
议题三:合成多肽药物的药典标准及更新
Compendial standards and program updates for therapeutic synthetic peptides
议题四:合成多肽药物的 GMP 生产与法规
GMP manufacturing and regulations
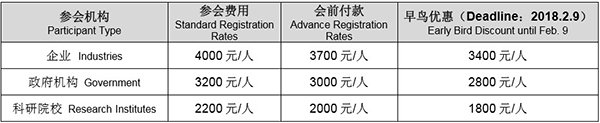
参会费 Conference Pricing:
报名方式 Online Registration:
在线报名:www.echinachem.com/events/2018usp/register.html
联系人 Contact: 王艳红 / Nina Wang
手机:13884941886(微信同号)
Email:yanhong.wang@echinachem.com





